John Tomich, Ph.D., Professor
Director of Biotechnology/Proteomics Core Facility
Undergraduate Advisor
Major research interests
Our lab is quite diversified with regard the synthetic, analytical, physical and electrophysiological approaches we employ to study our biological system. Four mass spectrometers (MALDI-TOF, LC-ESI-Ion Trap (also APC ionization), Nanoflow LC-ESI-Ion Trap, MALDI TOF/TOF) are housed in my lab along with peptide synthesizers, peptide sequencers, HPLCs, UV/Vis and Fluorescence spectrometers, BiaCore 3000 Plasmon Resonance instrument, and bilayer and patch clamp set-ups. We are also heavy users of the NMR facilities at K-State (500 MHz) as well as Kansas University (800 MHz). We are also experienced in analytical ultracentrifugation, circular dichroism and various calorimetry techniques.
Synthetic Anion Channels
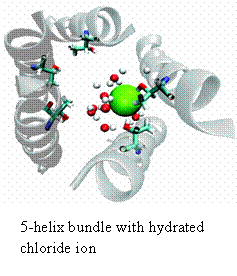
For almost all of my research career I have investigated the movement of ions across biomembranes using a reductionist model system. My early studies in collaboration with Mauricio Montal (UCSD) focused on identifying the pore-lining peptides from native channel proteins. During this period we defined the segments from numerous channels including the acetylcholine receptor (AChR), the glycine receptor (GlyR), a rat brain sodium channel, the cystic fibrosis transcellular conductance regulator (CFTR), and a human heart calcium channel. Members of the Acetylcholine Receptor superfamily of channels (that include the Glycine receptor) show the simplest geometries; that being a simple bundle of parallel helical segments comprised of 4 or 5 helical segments. Over the part 15 years we have focused on the channel properties of the second transmembrane domain of the glycine receptor (M2GlyR). This channel is chloride selective displaying a 25:1 monovalent anion: monovalent cation. In these studies, we have synthesized and tested more than 300 analogs of M2GlyR. Analogs showing reduced concentrations for supramolecular assembly and higher transport activities have been identified. The structures of the native and several high activity analogs have been solved using multi-dimensional solution NMR techniques. The pores are roughly 3 nm long and have an interior diameter of 0.6 nm. The different sequences are being tested as a possible therapeutic intervention for treating patients with cystic fibrosis. We have also been collaborating with a material sciences group at Johns Hopkins trying integrate our channels into simple microprocessor circuits. Most recently, we have begun to use computer simulations in conjunction with our NMR coordinates and model phospholipid bilayers to simulate the assembled structure of the pore and model the passage of chloride ions through the pore. We have identified the residues that appear to be involved in the anion selectivity filter: a vestibule formed by the ten beta-hydroxyls contributed by the threonines at positions 17 (shown in light blue in figure) and 13 (not shown). The goals for the next few years include- determining the stoichiometry of the helical segments that make the pore, studying the hydrogen bonding pattern of the pore lining hydroxyl groups with chloride and designing and building a planar non-peptidyl heterocylic compounds that position the hydroxyls as they occur in the selectivity filter(s) of the assembled pore.
Peptide Modulators of Tight Junctions in Polarized Cells
Both epithelial and endothelial cells come together and attach to one another to form barrier layers in animals. Examples of these are the air/tissue interface of the cornea or lung, the liquid/tissue interface in the digestive tract or the blood/brain barrier. The resistance level of these barriers varies from 60 to 20K Ohmcm-2 in different tissues. This barrier is a major impediment to drug delivery in a number of tissues. A subclass of pore-forming peptides was discovered while preparing the M2GlyR derivatives described above. A palindromic sequence based on the N-terminal 11 residues displayed an unusual property. In addition to forming a channel it caused monolayers of epithelium to lose resistance for a short period of time and in a reversible fashion. Six different epithelial monolayers were tested in vitro and all showed resistance loss to the peptide at concentrations of 60 mM. MDCK cells were repeated exposed and then wash free of the peptide for a period of several weeks. With each exposure the cells lost resistance. Upon removal of the compound the cells regained fully their resistance after 24 hr. The transport of large hydrophilic sequences was tested when the cells show reduced resistance. Blue dextrans with masses up to 70 KDa but not greater than 1.5 MDa were transported between the cells during the low resistance phase. We are now exploring potential applications of these peptides as short-acting modulators of the corneal epithelial barrier in the eye. We hypothesize that inclusion the palindromic sequence with this or other hydrophilic drugs should greatly enhance ophthalmic drug delivery. We have measuring transport of model compounds, including the anti-cancer drug methotrexate, across isolated cornea using mass spectrometer in the presence and absence of our highest potency peptide as well as several control sequences. The outcomes of these studies will identify specific target tissues in the eye that can be therapeutically modulated by this peptide as well as the areas of the eye that can be accessed using this approach.
Model Peptide Adhesives
Most commercial adhesives contain chemicals that are harmful to the environment. The development of safe bio-based adhesives could alleviate many of these harmful effects. Most protein adhesives work through receptors or cross-links to bring about adhesion. The new peptide adhesive motif developed in our laboratory requires no receptor or cross-links to achieve maximal adhesive strength. More than 20 peptides with different degrees of adhesive strength have been designed and synthesized using solid phase chemistries. All peptides contain a hydrophobic sequence flanked by positively or negatively charged amino acids trimers. The adhesive strength of the peptides in gluing wood strips was investigated at different pH values and hot press temperatures. The adhesive peptides self aggregate and interact with the wood surface. Based on these studies, a novel synthetic peptide was identified with high adhesive strength toward wood (dry: 3.7-4.0 MPa). The highest shear strength was observed at pH 12 for a sequence with only a five-residue hydrophobic core. The best adhesive peptide underwent structural analyses in water using circular dichroism, laser-FTIR, and laser desorption mass spectrometry. At pH 12 the most active peptide adopted a pH-induced beta-like conformation. Adhesive strength reflects contributions of both hydrogen bonding and van der Waals interactions. Ionic and covalent bonds do not appear to be significant factors. The sequence can also be charge neutralized at neutral pH by chemical methods. Our most recent data shows that these sequences are forming nanofibrils at pH 12.0 and that entanglement of the fibers is most likely the source of the adhesive properties. At low pH, these same sequences from nanoparticles and hollow nanospheres. We are currently evaluation the properties of these new structures.