Rafferty Lab Research "Pillars"
|
|
||||
|
|
|
|
||
Click below to learn more about research aspects of our lab.... |
||||
|
|
|
|
||
 |
Back to Top |
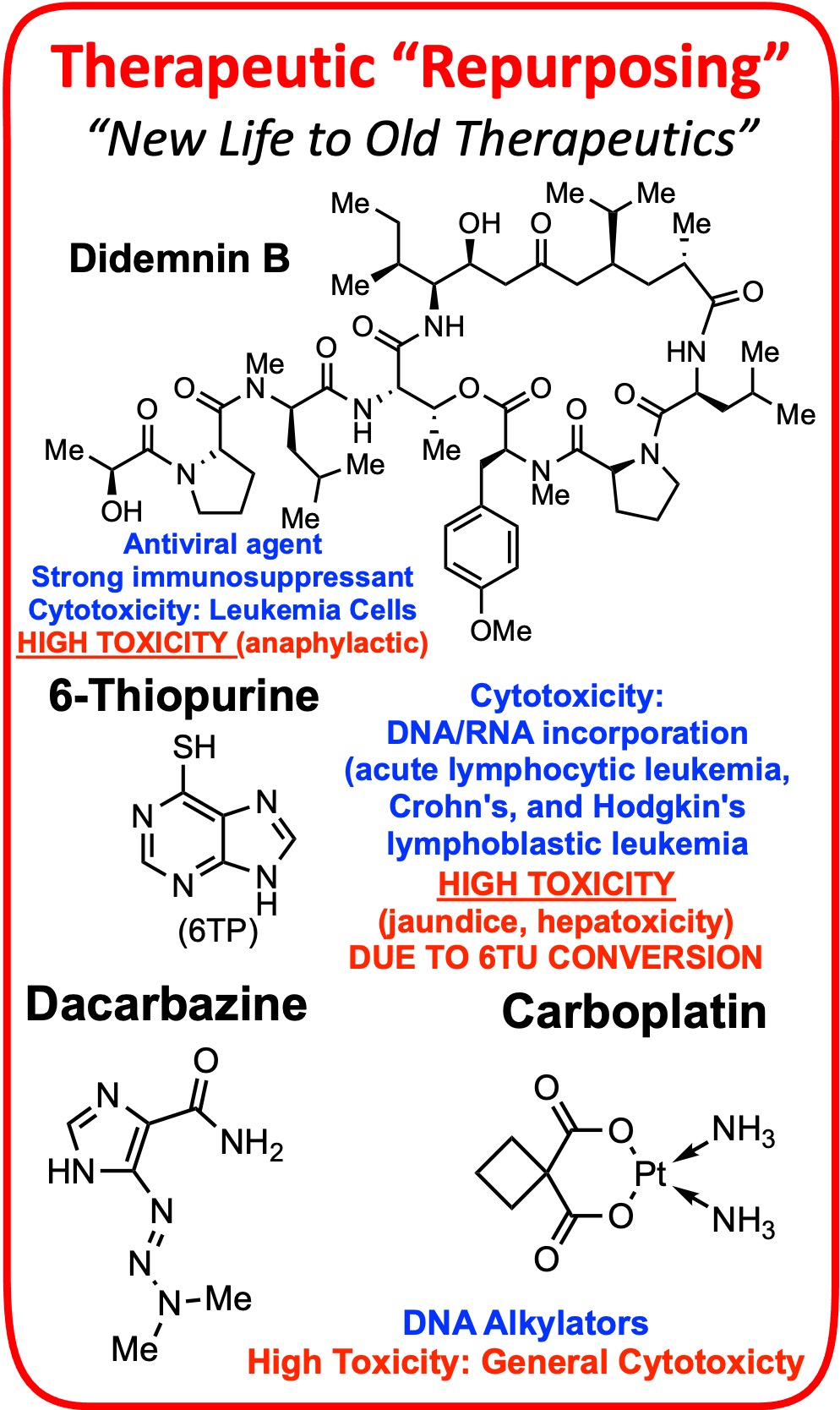
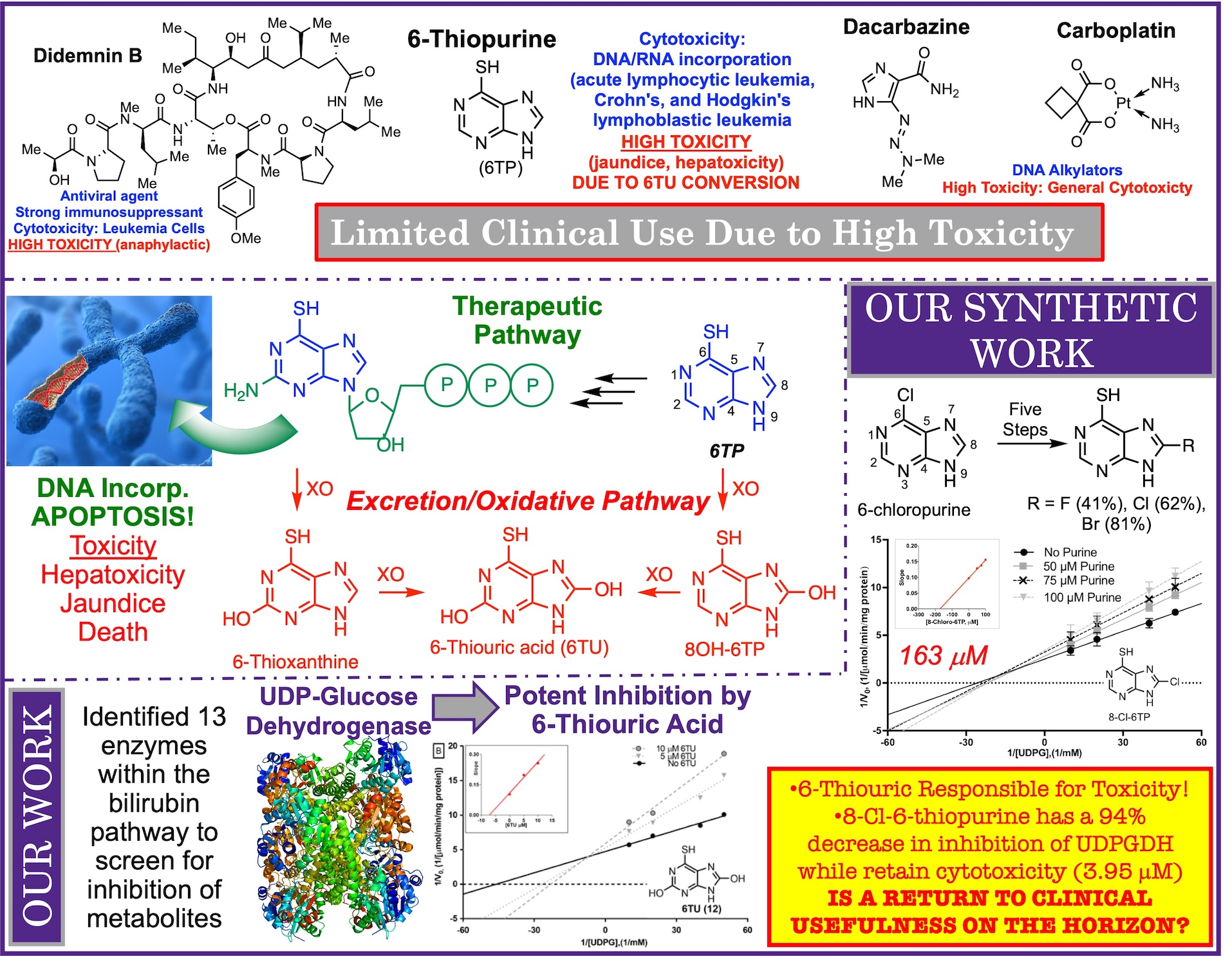
Countless small molecules and potential drugs fail to reach the market due to their off-target toxicities. In other cases, clinicially approved drugs are severely limited in their use for the treatment of diseases due to their toxicities. In this research pillar, we aim to take these potentially game changing drugs and elicit their toxicity pathways, and to use this information to redesign the parent compound/drug to eliminate its toxicity while retaining its potential game changing cytotoicity. Below, is our story behind the redesign of 6-thiopurine.
 |
 |
 |
|
 |
|
 |
 |
Back to Top |
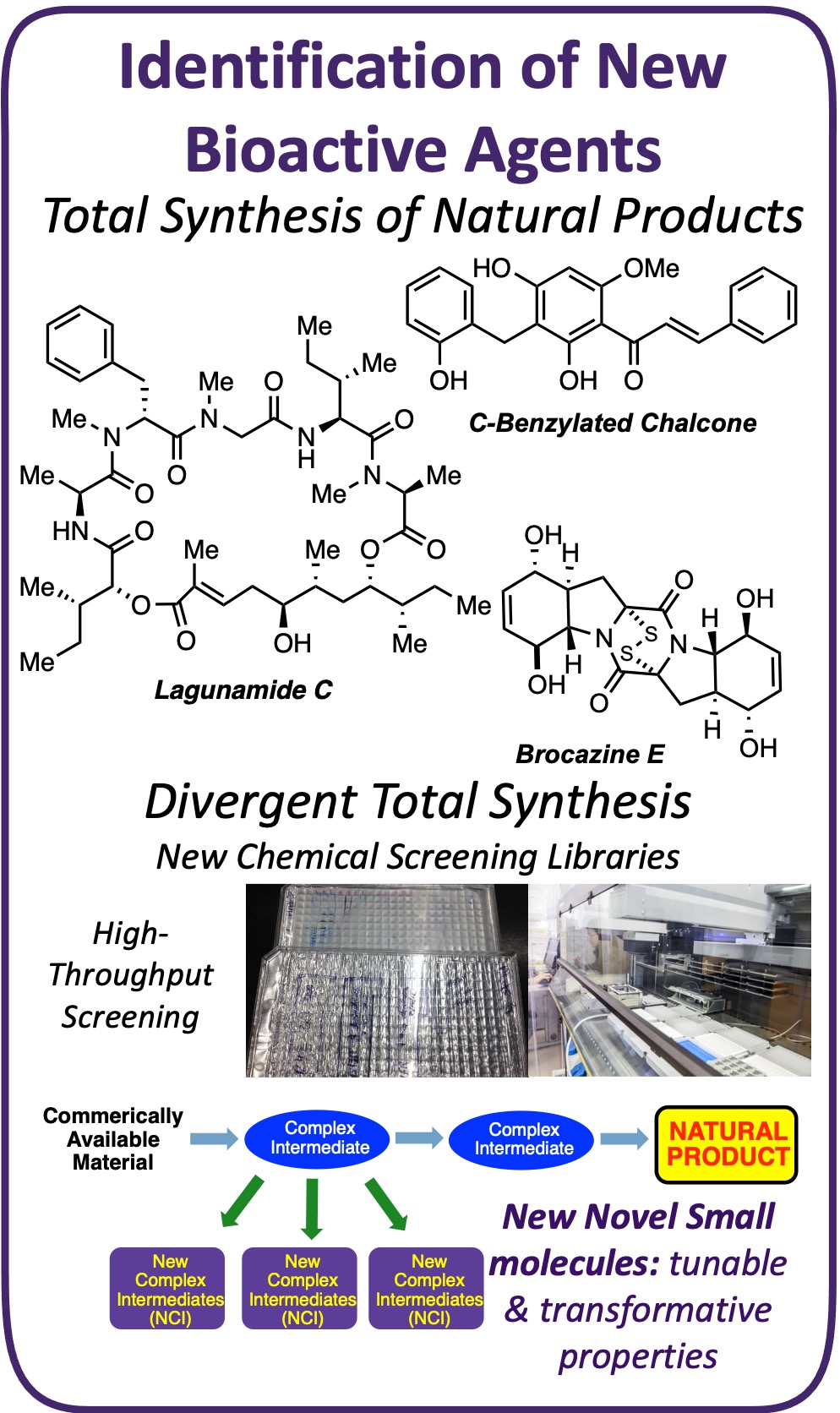
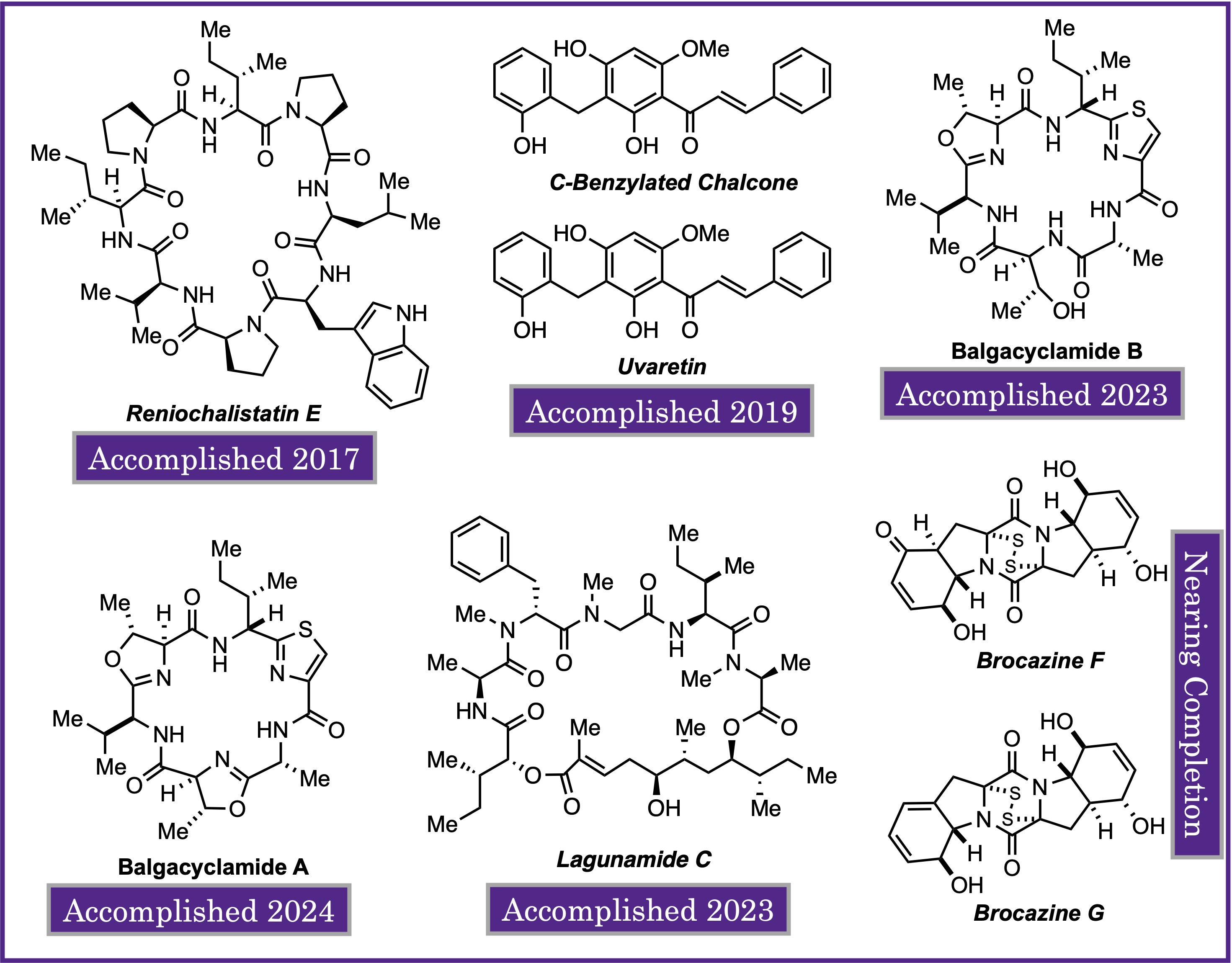
Natural products continue to be a dominant force in the discovery and the development of new therapeutic agents; as sole identities, derivatives, or serve as synthetic inspiration for new active pharmaceutical ingredients (APIs). In this research pillar, our lab focuses on the total synthesis of synthetically interesting and biological activity natural products (NPs). The NPs also have synthetic cores, or modules, that can be exploited in our labs chemical library synthesis (see below). We have completed the total sythesis of multiple natural products. Rather than this being the end of their journey, in our lab we aim to take all natural products accessed and push them to their full therapeutic potential. This includes screening as potentiating agents, transformation into drug delivery vehicles, and more.
Natural Product Total Synthesis
 |
 |
 |
|
 |
|
 |
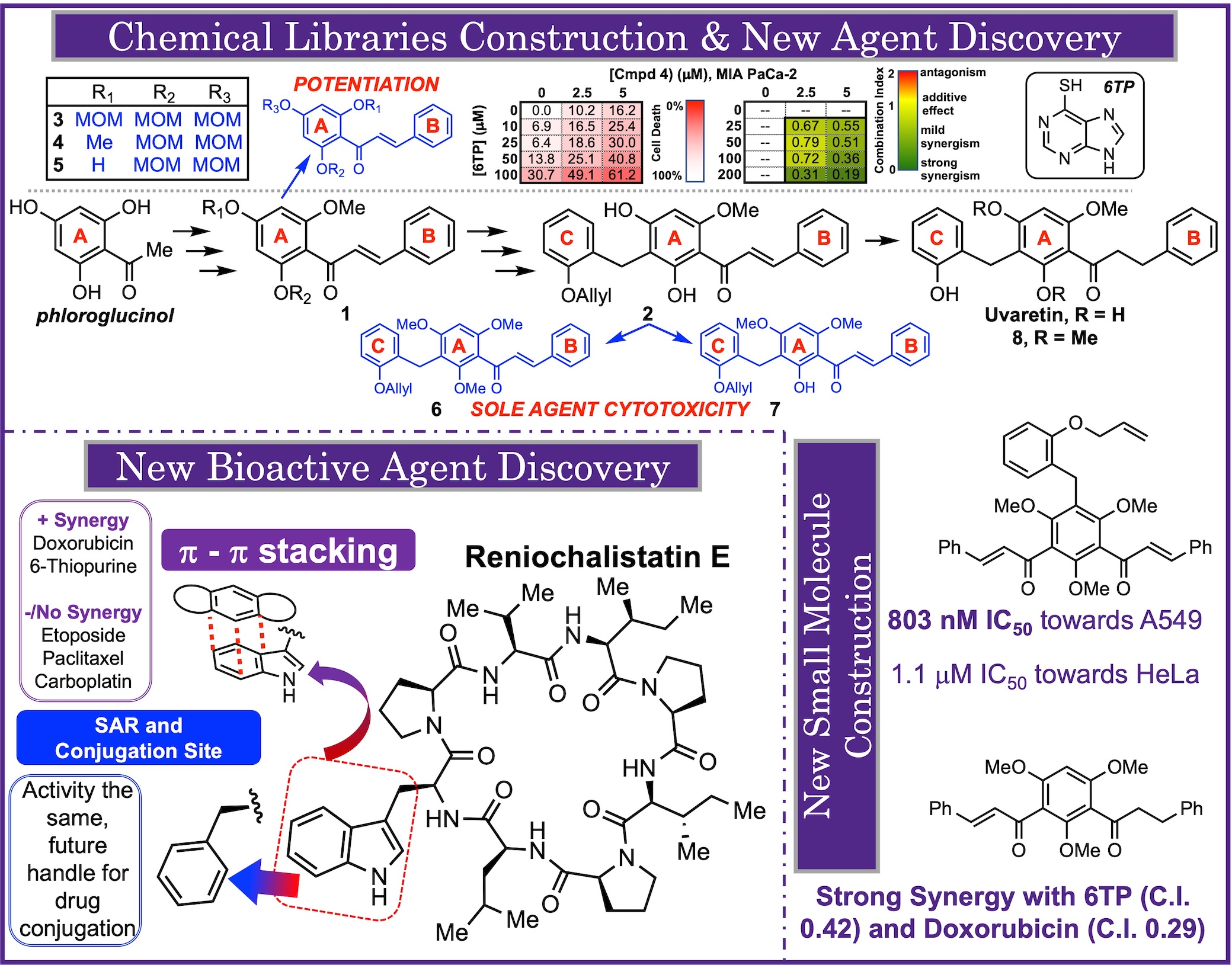
High-Throughput Screening (HTS) first entered the drug discovery field in 1986, in which the chemical screening library (CSL) plates are employed to help identify new APIs, new fragments, and so much more. The chemical screening plates are the most important part of HTS. While natural products, and their total syntheses, has been the hallmark in drug discovery, the population of chemical screening plates by NPs alone is not achievable. To solve this, these plates were populated with flat compounds, accessed via sp2-sp2 couplings, which has led drug discovery in decades of "flatlandness", this resulted in a decrease in new potent therapeutic agent discovery. To date, many new strategies have been adopted to help take HTS out of "flatlandness", many focused on the structural modification of natural products (Hergenrother's Complexity to Diveristy) and library construction from a chiral scaffold (Schreiber's Diversity Orientated Synthesis).
In this research pillar, we aim to construct new chemical screening libraries rooted in NPs. Rather than chemical modifying NPs themselves, we aim to take the complex and synthetic rich intermediates in our routes as the building blocks for new CLS compound members. Our intermediates will are subjected toward structural remodeling in the efforts of generating new interesting structures of varying biological properties. To date, we have illustrated the strong potential of this strategy through the chemical library formation via our labs total synthesis of uvaretin. Numerious library members were discovered with sole agent biological properties, as well as others having potentiating properties with current therapeutic agents used in the clinic. In addition to our CSL work, we also aim on exploring how to take our NPs and transform them into new drug delivery vehicles. Our work with reniochalistatin E is an example of this, in which this NP can be employed to delivery approved therapeutic agents via a unique pi-pi stacking conjugation.
Chemical Library Construction and New Bioactive Agent Discovery
 |
 |
 |
|
 |
|
 |
 |
Back to Top |
 |
 |
 |
|
 |
|
 |
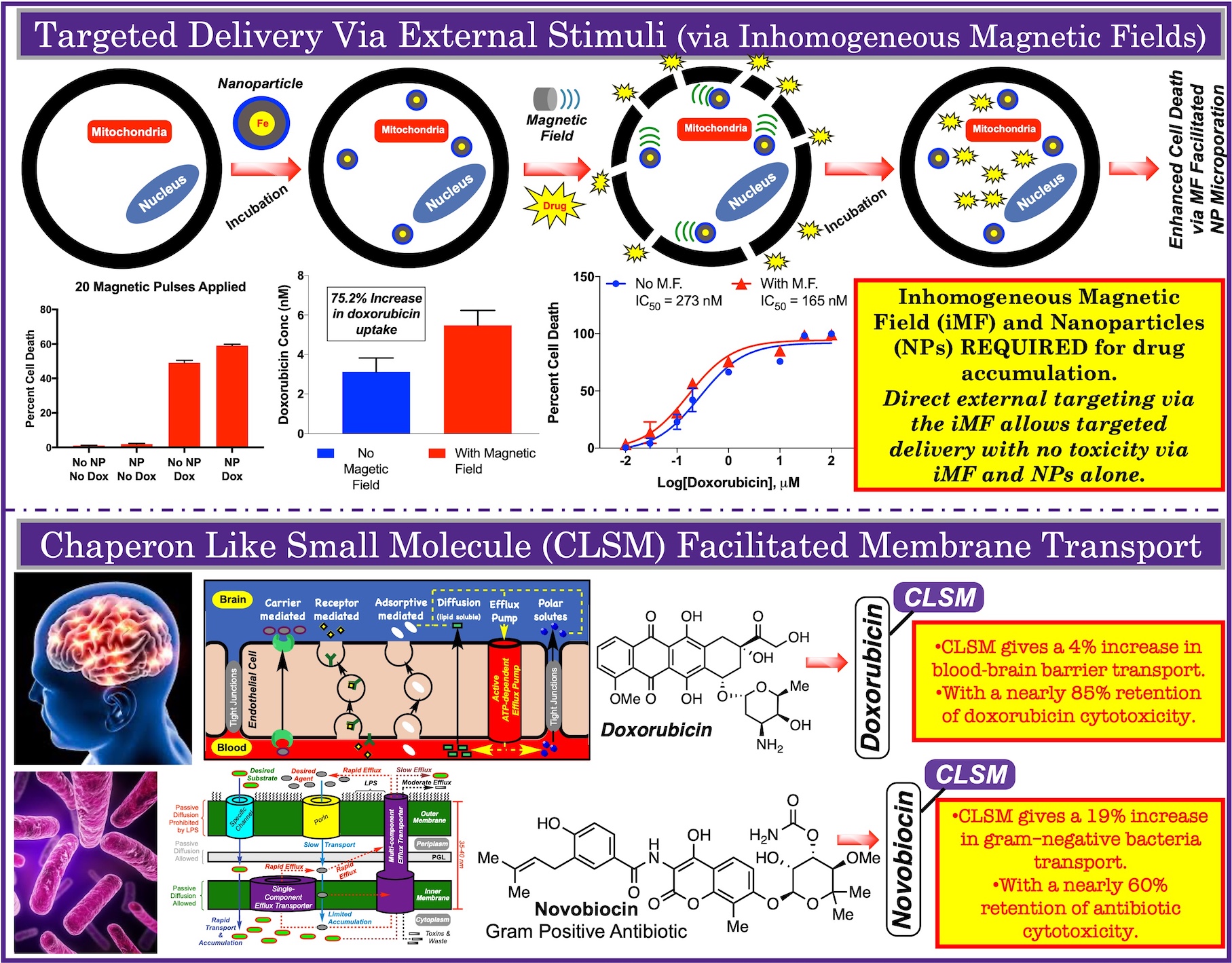
Treatment of brain cancers remains one of the main challenges in oncology. The three major types of brain cancer, all classifications of gliomas, are: astrocytomas, oligodendrogliomas, and oligo-astrocytomas. Current standard of care consists of surgical removal, radiotherapy, and/or chemotherapy. Unfortunately, prognosis for patients with malignant brain tumors is poor. Surgical removal increases median survival by 20 weeks, radiotherapy incorporation extends to 36 weeks, and inclusion of chemotheraphy increases survival to 40-50 weeks. The failure to treat these tumors is due in part to the inability to deliver therapeutics across the blood-brain barrier (BBB).
The BBB is a formulation of multiple structural, cellular (endothelial, astrocyte and pericytes cells), and physiological components that govern movement of molecules and ions. Further protecting the brain, the cellular structure of the BBB is held together and guarded by tight junctions (TJ), forming a diffusion barrier. Presences of these junctions require that small molecules enter the brain through these cells, not between. In addition, the presence of multidrug resistant proteins (MRPs) and P-glycoproteins (P-gp), located at the apical side of the BBB, actively pump a variety of anticancer agents (e.g. paclitaxel and anthracyclilnes) back into the blood.
Studies have shown that the chemophysical properties of polar surface area (tPSA) and cLopP contributor a factor into penetration. While these properties aid in preliminary calculations to predict penetration, they fail to explain other effects and their roles in penetrating this complex barrier. Noting that glycylglycine fails to penetrate, but the cyclic/rigid form is penetrable suggests that rigidity is also an important property for penetration, along with others.
To investigate the various chemophysical properties roles in BBB penetration through the synthesis of a small molecules varying in single properties. Evaluation of compounds will be performed using a parallel artificial permeability assay, which mimics the BBB tight and adherent junctions. Scaffolds that penetrate the BBB-mimic will be compared evaluated in vivo for penetration and efficacy.
| Back to Top |