December 18, 2014
Research aims to improve rechargeable batteries by focusing on graphene oxide paper
Submitted by Communications and Marketing
A Kansas State University engineering team has discovered some of graphene oxide's important properties that can improve sodium- and lithium-ion flexible batteries.
Gurpreet Singh, assistant professor of mechanical and nuclear engineering, and Lamuel David, doctoral student in mechanical engineering, India, published their findings in the Journal of Physical Chemistry in the article "Reduced graphene oxide paper electrode: Opposing effect of thermal annealing on Li and Na cyclability."
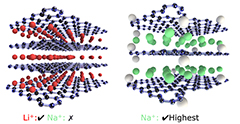
Graphene oxide is an insulating and defective version of graphene that can be converted to a conductor or a semiconductor when it is heated. Singh and his team studied graphene oxide sheets as flexible paper electrodes for sodium- and lithium-ion batteries.
The researchers found that sodium storage capacity of paper electrodes depends on the distance between the individual layers that can be tuned by heating it in argon or ammonia gas. For example, reduced graphene oxide sheets, or rGO, produced at high temperature have near zero sodium capacity, while reduced graphene oxide sheets produced at 500 degrees C have the maximum capacity.
"The observation is important because graphite, which is a precursor for making graphene oxide, has negligible capacity for sodium and has long been ruled out as viable electrode for sodium-batteries," Singh said. "Graphite is the material of choice in current lithium-ion batteries because the interlayer spacing is just right for the smaller size lithium ions to diffuse in and out."
The researchers are the first to show that a flexible paper composed entirely of graphene oxide sheets can charge and discharge with sodium-ions for more than 1,000 cycles. Sodium perchlorate salt dissolved in ethylene carbonate served as the electrolyte in their cells.
"Most lithium electrode materials for sodium batteries cannot even last for more than a few tens of charge and discharge cycles because sodium is much larger than lithium and causes enormous volume changes and damage to the host material," Singh said. "This design is unique because the distance between individual graphene layers is large enough to allow fast insertion and extraction of the sodium ions, thanks to the oxygen and hydrogen atoms that prevent sheets from restacking."
Singh and his team also studied the mechanical behavior of the electrodes made of reduced graphene oxide sheets. The researchers measured the strain required to tear apart the electrodes. Through videography, they showed the ability of the crumpled graphene oxide papers to sustain large strains before failing.
"Such measurements and study of failure mechanisms are important for designing long-life batteries because you want the electrode to be able to expand and contract repeatedly without fracture for thousands of cycles, especially for larger nonlithium metal-ion batteries," Singh said. "These days, almost every one is using crumpled graphene as either the conducting agent or elastic support or both."
Earlier this year, Singh and his team demonstrated large-scale synthesis of few-layer-thick sheets of molybdenum disulfide. They also showed the molybdenum disulfide/graphene composite paper has potential as a high-capacity electrode for sodium-ion battery. In that research, the scientists used graphene as an electron conductor for the molybdenum disulfide sheets and observed graphene to be largely inactive toward sodium.
Their latest research has shown that unlike sodium, the lithium capacity of rGO increases with increasing rGO synthesis temperature reaching maximum value for sample produced at 900 degrees C.
"It is only now we realize that sodium capacity of graphene, or rGO, is dependent on its processing temperature," Singh said. "The rGO specimens in our previous study were prepared at 900 degrees C."
Singh said that research into sodium and nonlithium batteries is important for several reasons. As the focus shifts from vehicles to stationary energy storage systems and large vehicles, stationary batteries need to be cheaper, safe and environmentally benign. Because of its large abundance, sodium is a potential candidate for replacing lithium-ion batteries.
By focusing on nanotechnology, Singh and his team were able to explore and design materials that can store sodium-ions reversibly and without damage. They found their answer in graphene oxide, which can cycle sodium-ions for more than 1,000 cycles.
Singh and his team will continue exploring new nanomaterials and focus on materials that can be mass-produced in a cost-effective manner.
"We would like to perform fundamental studies to understand the origins of first cycle loss, voltage hysteresis, and capacity degradation that are common to metal-ion battery anodes prepared from 2-D layered crystals such as transition metal chalcogenides, graphene, etc.," Singh said.
The researchers are also looking at other nanomaterials that have been ruled out as battery electrodes, such as boron nitride sheets and silicon-nitrogen based ceramics.
Singh has received support from the National Science Foundation.
